Brain organoids: defining maturity, addressing the challenges and exploring future directions
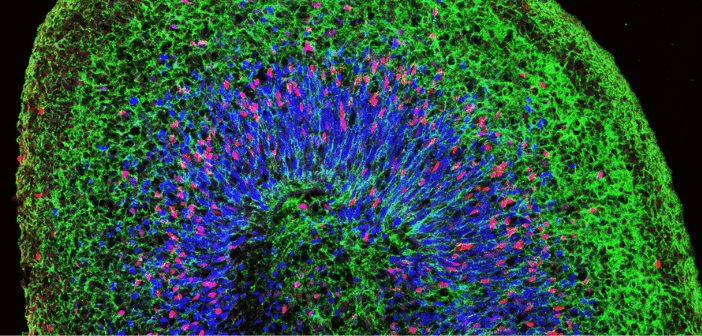
Credit: UCLA Broad Stem Cell Research Center/Cell Reports
Alysson Muotri is a Professor at the School of Medicine at the University of California in San Diego (CA, USA). His research focuses on modeling neurological diseases, such as autism spectrum disorders, using induced pluripotent stem cells. Alysson's lab has developed several techniques to culture functional brain organoids for basic research and drug-screening platforms. In this interview, Alysson speaks to us about his work with organoids to study the mechanisms of brain development. He also discusses the challenges he faces when using organoids in his work, such as automation, optimization and vascularization, and how we might address these obstacles. 1.Your...