CLICK-MS and MASTER-2 Phase IV trial design: cladribine tablets in suboptimally controlled relapsing multiple sclerosis
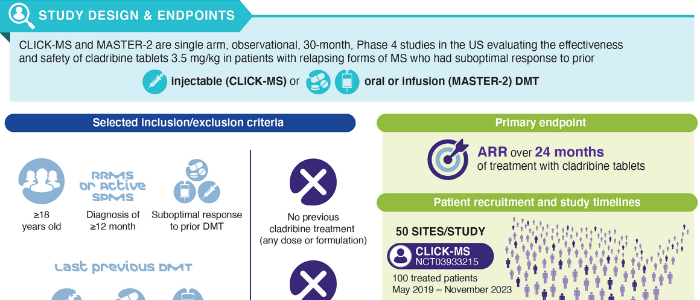
CLICK-MS and MASTER-2 are ongoing Phase IV clinical trials evaluating cladribine tablets in suboptimally controlled relapsing multiple sclerosis. These trials will help to add real-world data to the body of existing evidence on the use of cladribine tablets, which have already been approved for the treatment of relapsing forms of multiple sclerosis. The trials are expected to conclude in 2023.
The latest Clinical Trial Protocol published Neurodegenerative Disease Management reviews the rationale and design of the CLICK-MS and MASTER-2 clinical trials.
Abstract: Cladribine tablets 10mg (3.5mg/kg cumulative dose over 2 years) are approved for the treatment of relapsing forms of multiple sclerosis (MS), including relapsing–remitting MS (RRMS) and active secondary progressive MS (SPMS). However, real-world data on cladribine tablets are limited. CLICK-MS and MASTER-2 are single arm, observational, 30-month, Phase IV studies in the USA evaluating the effectiveness and safety of cladribine tablets 3.5mg/kg in patients with RRMS or active SPMS who had suboptimal response to prior injectable (CLICK-MS), or infusion/oral (MASTER-2) disease-modifying therapy. The primary endpoint is 24-month annualized relapse rate. Key secondary endpoints include patient-reported outcomes on quality-of-life measures, treatment adherence and adverse events. Studies began in 2019 and are expected to be completed in 2023.
Clinical Trial Registration: NCT03933215 and NCT03933202 (ClinicalTrials.gov).