Ask the Experts: precision medicine for neurological diseases

In this ‘Ask the Experts’ series, we’ve brought together a panel of key thought leaders to discuss the use of precision medicine for neurological diseases. What progress has been made over the last few years? What are the challenges surrounding the implementation of precision medicine? How might the incorporation of precision medicine into clinical practice be better streamlined?
Our experts for this instalment include Alberto Espay (University of Cincinnati, OH, USA) and Maria Teresa Ferretti (Women’s Brain Project, Zurich, Switzerland). Take a look at the full discussion below to hear their insights.
What progress has been made in precision medicine for neurological diseases over the last 5 years?
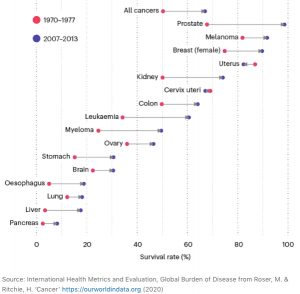 Alberto Espay: There has been virtually no progress in this area. Every single trial conducted to date with the purpose to slow down progression has been negative. The reasons used to justify the lack of benefits are invariably related to design or execution of the trials. There has been no reckoning about the possibility that, if indeed Parkinson’s disease is not truly a single disease (and we can say the same for Alzheimer’s, dementia with Lewy bodies, frontotemporal dementia, etc.), a single approach to therapy cannot possibly ever change the disease.
Alberto Espay: There has been virtually no progress in this area. Every single trial conducted to date with the purpose to slow down progression has been negative. The reasons used to justify the lack of benefits are invariably related to design or execution of the trials. There has been no reckoning about the possibility that, if indeed Parkinson’s disease is not truly a single disease (and we can say the same for Alzheimer’s, dementia with Lewy bodies, frontotemporal dementia, etc.), a single approach to therapy cannot possibly ever change the disease.
To this end, I wrote this note on my Twitter account recently: “What improved outcomes in #Cancer after 1977, eluding its biological opposite, #Neurodegeneration? Cancers were divided into (and targeted as) genetic-molecular subtypes. We are still trying to explain #Parkinsons and #Alzheimers as “heterogeneous” but unique disorders.” (Based on the image to the top right, published in Nature).
Maria Teresa Ferretti: In comparison to other disciplines such as oncology, precision medicine is in its relative infancy in neurology. This is rapidly evolving; the difficulty in diagnosing patients as well as the common lack of efficient treatments has highlighted the limitations of classical “shallow” medicine, to quote Eric Topol (Scripps Research Institute, CA, USA) [1].
However, research in the past 5 years has resulted in significant changes in neurological clinical practice. In the field of Alzheimer’s disease (AD), which is my field and where the Women’s Brain Project (WBP) focuses in particular, this is very clear.
In particular, we now know that patients are extremely heterogeneous: two patients with the same symptom (let’s say dementia) might have two completely different underlying pathologies. Vice versa, the same brain lesion (for instance, accumulation of amyloid plaques) in two patients can result in different presentations – some most happily carry amyloid plaques until their death with no signs of cognitive decline, and some progress within a matter of years [2,3].
Given such heterogeneity, a one-size-fits-all approach has low chances of success in both diagnosis and therapy. It was only a few years ago that we realized that up to 30% of AD patients could not possibly benefit from anti-amyloid drugs as they did not have amyloid [4]. As a result, contemporary clinical trials for anti-amyloid agents in AD, such as the ones for aducanumab [5] and gantanerumab, pre-select patients based on amyloid positivity [6].
In the field of AD alone, there is a whole pipeline of treatments that are not directed to a generic AD patient but designed against specific hallmarks. Anti-tau and anti-inflammatory treatments are clear examples [6]; these will be administered only to the patients expressing biomarkers of tau or inflammation, respectively. This is tremendous and much needed progress.
What promising avenues are being developed in the field?
Maria Teresa Ferretti: The implementation of biomarker-based diagnoses holds great promise. Unfortunately, the use of clinical signs and neuropsychological assessment for differential diagnoses, which is gold standard for the diagnosis of dementia, is associated with delays and high error rates.
In contrast, the implementation of specific biomarkers (in blood, cerebrospinal fluid and neuroimaging) allows for a precise, timely and accurate diagnosis, which can then inform therapeutic decisions.
Even though such biomarkers in AD are not yet implemented in clinical practice, they are extensively studied and characterized in research settings, and will be soon ready for prime time [7]. These will include markers of inflammation and neurodegeneration such as in the Neurotool kit, which is under development by a consortium of stakeholders [8].
Alberto Espay: The most radically different avenue being examined is based on the biophysical properties of all brain proteins. Indeed, biophysical experiments demonstrate that brain proteins function normally only in their native, soluble state. Upon exposure to nucleating surfaces, they undergo polymerization – a process similar to crystallization in which there is a physical transformation from soluble to insoluble, and a biological transformation from functional to nonfuctional. The marked stability of the resulting cross-β conformation of fibrils precludes the toxicity and virus-like replication attributed to them.
The early neuronal toxicity in neurodegenerative disorders may arise not from the accumulation of aggregated proteins in a polymerized state but from a reduction in the soluble, normal-functioning precursor. This alternative loss-of-function hypothesis deserves to be tested by shifting efforts from insoluble protein destruction (as with several ongoing anti-amyloid and anti-synuclein monoclonal antibody trials) to soluble protein replacement. This will not be quite precision medicine but as ‘rescue medicine’, it will stand to form the foundations for such future. Precision medicine will truly require identifying the reasons why proteins polymerize by identifying the so-called nucleating surfaces. These are the true pathogens that force the liquid-to-solid phase transformation of brain proteins, which presumably vary across individuals.
What are the challenges surrounding the implementation of precision medicine for neurological disorders? What are the main barriers hindering progress?
Alberto Espay: The major challenge is the idea that if we continue to study large cohorts of affected people, we can create the knowledge we need to move into precision medicine. Unfortunately, the averages of diseases are truly applicable to virtually no one. If each disease is made of many biological disruptions, whatever is common to them all cannot be pathogenic to each individual. The greatest distraction from progress is the blind embrace of aggregated proteins as the cause of the problem. I learned from Kariem Ezzat (Karolinska Institute, Solna, Sweden) that proteins are not acting like viruses, but acting like proteins. They polymerize, not ‘virulize’, as we have come to conceive them in ideas such as ‘replication’ and ‘prion-like spread’.
Maria Teresa Ferretti: A main challenge is the education of medical care providers in neuropathological hallmarks of brain diseases, reflecting a lack of appreciation for the heterogeneity of the patients and underlying pathologies. The need to clearly identify patients in order to provide them with the best possible treatment is also not clear.
Many argue that, not yet having an effective treatment, there’s no point in pushing for a molecular and precise diagnosis. I would argue the opposite. If we do not have a treatment yet, it is precisely because we lack a precision medicine approach. We have been designing trials involving patients who actually had different diseases, so we had no chance to find a signal. Once this mindset is changed, the next challenge will be practical [9].
In your opinion, how might these challenges be overcome?
Maria Teresa Ferretti: We need to advocate, publish papers and introduce precision medicine into the curriculum of healthcare providers. This could be done with crosspollination from different disciplines (e.g., oncology), where precision medicine is much more advanced.
This is also part of the work we do at WBP – we raise awareness on the heterogeneity of patients, starting with sex and gender differences. We believe that differences in disease presentation between men and women, which are increasingly recognized by the medical community, are the gateway to precision medicine [10,11].
Alberto Espay: Nothing short of a major challenge from the outside world will change things for the better. We neurologists are the most traditionalist and protective of medical species. The most important breakthrough we need is a cognitive one. If we can accept that Parkinson’s is not one disease and that Alzheimer’s is not one disease, we can abandon the hopeful but illusory idea of a “cure for Parkinson’s” or a “cure for Alzheimer’s”. We can only cure small groups of biologically identifiable individuals at a time, not diseases that are not diseases, but syndromes. There is an uphill battle ahead as most ongoing studies are aiming at slowing the progression of full diseases, not molecular subtypes thereof.
How could artificial intelligence be better harnessed to improve our understanding of each individual’s condition?
Maria Teresa Ferretti: To characterize an individual’s condition in AD, we would need to consider at least genetic factors (e.g., APOE), phenotypic factors (hallmarks of disease), gut microbiome, demographics such as age and sex, and comorbidities.
Such multidimensional datasets are impossible to grasp for humans but are perfect for the application of artificial intelligence-powered predictive algorithms. Furthermore, I strongly believe that artificial intelligence will be increasingly used to support clinical diagnosis and tracking of symptoms [12], as it has done with multiple sclerosis [13].
This represents a huge step forward compared with clinical evaluation, which is prone to human bias and lacking the temporal dimension.
How might precision medicine provide substantial benefit to patients with neurological diseases?
Alberto Espay: Precision medicine can only be enacted if we knew the molecular and biological characteristics of the patient to be targeted. That is not the case for the vast majority of patients with neurodegenerative disorders. For some, we have knowledge of their genetic mutation, but even the genetic abnormality is not deterministic as to the type of biological change it may be associated with. For instance, it is well known that both LRRK2 and GBA, two very common genetic causes of Parkinson’s, can be associated with a range of pathologic features, including no Parkinson’s.
Maria Teresa Ferretti: The advantages of precision medicine from a patient perspective became crystal clear when I had to face breast cancer 18 months ago. It took exactly 1 week for me to have the first diagnosis, 2 weeks to have the molecular characterization of my tumor and consequent therapeutic decision, and a grand total of 6 months for the surgery and the start of radio- plus hormonal therapy. Using a predictive algorithm based on a panel of genetic markers [14], chemotherapy was deemed unnecessary, sparing me from months of suffering.
For me as a patient, precision medicine meant a precise diagnosis in 2 weeks, a clear therapeutic path tailored to my specific tumor, and the avoidance of an unnecessary treatment that could be more harmful than beneficial.
These are benefits that we must provide to neurological patients, who wait for up to 3 years [15] for a diagnosis and are often treated with drugs that are ineffective and give side effects.
Giving the right treatment to the right patient at the right time means reducing the personal suffering of the patient and his/her family, as well as a dramatic reduction in public socioeconomic costs [16], which, for neurological diseases, are massive.
In what ways could precision medicine impact current diagnosis and treatment of neurological disorders?
Alberto Espay: If we could understand each individual’s ‘biotype’, we could start applying the principles of precision medicine long deployed in other fields of medicine. A neurologist of the future will hopefully diagnose a patient with ‘Parkinson’s disease’ at the bedside but will quickly follow that by stating that such diagnosis means little until several tests are conducted to define the type and extension of disease. Only then, would we know how to treat that ‘brand’ of Parkinson’s. “While we wait for that, let’s try some levodopa to alleviate your symptoms”, might such neurologist also say.
How might the incorporation of precision medicine into clinical practice be better streamlined?
Alberto Espay: By enrolling as many patients as possible into a longitudinal cohort to study their biology long term. In Cincinnati (OH, USA), we have begun such approach. We have recently launched the Cincinnati Cohort Biomarker Program, which is the first phenotype-agnostic longitudinal cohort in neurodegenerative diseases. Phenotype-agnostic means all analysis are not anchored on the symptoms but on the biology. We are not asking, “what is different between people with versus without tremor” but rather, “who are the individuals who have this mitochondrial marker reduced two standard deviations below the mean?” and “who are those positive for the bioassay of X therapy, available for repurposing?” The latter aspect is a key component. We are not interested in simply determining the many biological subtypes of disease but in identifying those who could benefit by virtue of a link between their biology and the mechanism of action of a therapy already available.
A parallel effort will be to develop serum bioassays for such future repurposing efforts in biologically suitable individuals. Although there are many anticipated (and unforeseeable) challenges, this study aims at “walking the talk” in terms of moving from learning about diseases to learning about people with diseases. It deliberately abandons clinicopathologic criteria as the anchor for disease classification in favor of biomarker-driven disease subtyping, with an eye on future subtype-specific disease-modifying therapy. We have recently submitted for peer review an article about the design and methodology aspects of the Cincinnati Cohort Biomarker Program study.
Maria Teresa Ferretti: Precision medicine tools must be optimized and scaled for large use in the general population. The classic example is a blood biomarker for amyloidosis in AD patients, which would allow mass screening of thousands of patients, as opposed to the current PET scan and cerebrospinal fluid analysis. Further, safe storage systems for personal data have to be implemented. Finally, validated algorithms should be made available to the larger community and become an integral part of the neurologist’s toolkit, in the frame of a larger renovation of medicine.
What advancements are required to progress the field of precision medicine further in relation to neurological diseases?
Maria Teresa Ferretti: We need more data – from observational cohorts, real-world data and from clinical trials – to train diagnostic and predictive algorithms. In fact, carrying a single biomarker, such as β-amyloid, does not help much. Some people remain amyloid positive and perfectly sharp for decades, while others decline within a few years.
How factors such as genetic, phenotypic, comorbidities or demographic and lifestyle elements modulate the effect of a given biomarker, and how multiple biomarkers interact between each other, is still poorly understood.
In this regard, at WBP we are particularly passionate about the effect of biological sex in Alzheimer’s. Amyloid positivity has been linked to faster cognitive decline [17] and higher tau pathology [18] in women, which might account for at least part of the heterogeneity observed in amyloid-positive individuals.
Better and scalable biomarkers, with sex adjustments, are needed for molecular diagnosis. Further, clinical scales should be increasingly substituted by digital tools, allowing longitudinal, continuous and unbiased monitoring of a variety of functions, from motor to language.
Looking ahead, where do you anticipate the field of precision medicine will be for neurological diseases in the next 5–10 years?
Maria Teresa Ferretti: 10–20 years ago, breast cancer patients were all treated with chemotherapy. Some of them benefitted from it, some did not and succumbed to terrible side effects. It took years to learn that the same symptom can be caused by a variety of lesions with specific molecular and genetic makeup, which also had an impact on the specific response to treatment. Precision medicine promises to solve this.
Today, oncological diagnoses can be treated with a precision medicine approach [19]. My hope is for neurology to be in 10 years where oncology is today.
This will include having identified and characterized subgroups of patients (based on extensive genetic, phenotypic and clinical evaluation, which is standard practice), and having discovered specific treatments for at least half of them.
Meet the experts
 Alberto J Espay (MD, MSc, FAAN, FANA)
Alberto J Espay (MD, MSc, FAAN, FANA)
Alberto Espay is Professor and Endowed Chair of the James J and Joan A Gardner Center for Parkinson’s disease at the University of Cincinnati (OH, USA). He trained in Neurology at Indiana University (1998–2001) and in clinical and electrophysiology of Movement Disorders at the University of Toronto, Canada (2001–2005). He has published over 250 research articles and book chapters, including five books, among them Common Movement Disorders Pitfalls (Cambridge, Highly Commended BMA Medical Book Award, 2013) and Disorders of Movement (Springer, 2016). He has served as Chair of the Movement Disorders Section of the American Academy of Neurology, Associate Editor of Movement Disorders and in the Executive Committee of the Parkinson Study Group. He currently serves the International Parkinson and Movement Disorders Society as Chair of the Task Force on Technology and as Secretary of its Pan-American Section.
 His research efforts have focused on the measurement of motor and behavioral phenomena in, and clinical trials for, Parkinson’s disease as well as in the understanding and management of functional movement disorders. With colleagues at the University of Cincinnati and several international collaborators, he recently launched the first phenotype-agnostic biomarker study for neurodegenerative disorders (Cincinnati Cohort Biomarker Program) to deploy bioassays aiming at matching available therapies to disease subtypes most suitable to benefit from them, regardless of clinico-pathologic classifications. With Ben Stecher, a patient and Parkinson’s advocate, he wrote Brain Fables, scheduled for release by Cambridge in the summer of 2020.
His research efforts have focused on the measurement of motor and behavioral phenomena in, and clinical trials for, Parkinson’s disease as well as in the understanding and management of functional movement disorders. With colleagues at the University of Cincinnati and several international collaborators, he recently launched the first phenotype-agnostic biomarker study for neurodegenerative disorders (Cincinnati Cohort Biomarker Program) to deploy bioassays aiming at matching available therapies to disease subtypes most suitable to benefit from them, regardless of clinico-pathologic classifications. With Ben Stecher, a patient and Parkinson’s advocate, he wrote Brain Fables, scheduled for release by Cambridge in the summer of 2020.
 Maria Teresa Ferretti (PhD)
Maria Teresa Ferretti (PhD)
Maria Teresa is a neuroimmunologist and science advocate with over 10 years of international experience in the field of Alzheimer’s disease and a unique expertise on sex and gender differences.
After completing a Master’s degree in Pharmaceutical Chemistry in 2005 (University of Cagliari, Italy) and a stage at the Center of Excellence for Drug Discovery of GlaxoSmith&Kline (Harlow, England), she obtained a PhD in Pharmacology and Therapeutics at McGill University (Montreal, Canada) in 2011 with a thesis on the role of inflammation in early, pre-plaques stages of Alzheimer’s disease.
In 2011 she moved back to Europe and joined the Nitsch’s lab (University of Zurich, Switzerland) as a postdoc and group leader. As part of her research there, Maria Teresa led a small team, which employed single-cell, multidimensional deep immunophenotyping (with standard flow-cytometry and CyTOF) to identify novel biomarkers for increasing individual level prediction of cognitive decline and Alzheimer’s.
In 2016 Maria Teresa co-founded the non-profit organization ‘Women’s Brain Project’ (WBP, CHE-369.271.906), focusing on sex-sensitive precision medicine for brain and mental diseases such as Alzheimer, migraine, multiple sclerosis and depression, among others (www.womensbrainproject.com). Her work as WBP Chief Scientific Officer has led to several scientific publications in leading journals including Nature and PNAS, two TED-x talks and coverage by both the national (Sonntagszeitung, SwissInfo, NZZ, Le Temps) and the international press (including the BBC, The Independent, Sciences et Avenir, Financial Times, La Stampa, ELLE Italy).
Disclaimer
The opinions expressed in this discussion are those of the interviewees and do not necessarily reflect the views of Neuro Central or Future Science Group.
[1] Women’s Brain Project. “Deep medicine” – book review.
www.womensbrainproject.com/deep-medicine-book-review/
[Accessed 28 May 2020]
[2] Roberts RO, Aakre JA, Kremers WK et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 75(8), 970–979 (2018).
[3] Villemagne VL, Doré V, Bourgeat P et al. Aβ-amyloid and tau imaging in dementia. Sem. Nuclear Med. 47(1), 75–88 (2017).
[4] Rabinovici GD, Gatsonis C, Apgar C et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321(13), 1286–1294 (2019).
[5] Women’s Brain Project. Aducanumab is back for Alzheimer’s, and it might be a game changer.
www.womensbrainproject.com/aducanumab-alzheimers-gamechanger/
[Accessed 28 May 2020]
[6] Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. (N Y). 5, 272–293 (2019).
[7] Jack CR, Bennett DA, Blennow K et al. A/T/N: an unbiased descriptive classification scheme for Alzheiemr’s disease biomarkers. Neurology doi:10.1212/WNL.0000000000002923 (2016).
[8] Alzforum. Proteomics uncovers potential markers, subtypes of Alzheimer’s.
www.alzforum.org/news/conference-coverage/proteomics-uncovers-potential-markers-subtypes-alzheimers
[Accessed 28 May 2020]
[9] Rand Corporation. Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer’s treatment.
www.rand.org/pubs/research_reports/RR2503.html
[Accessed 28 May 2020]
[10] Ferretti MT, Santuccione-Chadha A, Hampel H. Account for sex in brain research for precision medicine. 569, 40 (2019).
[11] Ferretti MT, Iulita MF, Cavedo E et al. Sex differences in Alzheimer’s disease – the gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469 (2018).
[12] Women’s Brain Project. Digital biomarkers & the future of mental health.
www.womensbrainproject.com/digital-biomarkers/
[Accessed 28 May 2020]
[13] Nature Research. Digital health: smartphone-based monitoring of multiple sclerosis using Floodlight.
www.nature.com/articles/d42473-019-00412-0
[Accessed 28 May 2020]
[14] Breastcancer.org. Oncotype DX test.
www.breastcancer.org/symptoms/testing/types/oncotype_dx
[Accessed 28 May 2020]
[15] Woods B, Arosio F, Diaz A et al. Timely diagnosis of dementia? Family carers’ experience in 5 European countries. Int. J. Geriat. Psych. 34(1), 114–121 (2019).
[16] El-Hayek YH, Wiley RE, Khoury CP et al. Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J. Alzheimers Dis. 70(2), 323–341 (2019).
[17] Buckley RF, Mormino EC, Amariglio RE et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimers Dementia 14(9), 1193–1203 (2018).
[18] Buckley RF, Mormino EC, Rabin JS et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 76(5), 542–551 (2019).
[19] Moscow JA, Fojo T, Schilsky RL. The evidence framework for precision cancer medicine. Nat. Rev. Clin. Oncol. 15, 183–192 (2018).
You might also like:





