Carbon nanoparticles and oxidative stress: could an injection stop brain damage in minutes?
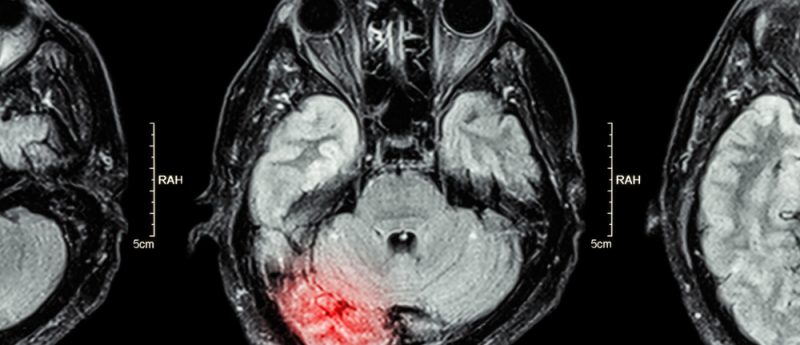
Reactive oxygen species (ROS), which include superoxide, nitric oxide, hydroxyl radicals, nitrogen dioxide and peroxynitrite, play a crucial role in vital processes such as blood pressure regulation, cell migration, neurotransmission, immune regulation, microorganism defense and smooth muscle relaxation [1]. Under normal conditions in healthy systems, ROS are regulated by enzymes including superoxide dismutase (SOD) and catalase, as well as by small molecule natural antioxidants such as glutathione, ascorbic acid, coenzyme Q10, vitamin A and vitamin E. The overproduction of ROS leads to a condition known as oxidative stress, and at elevated concentrations, ROS readily cause oxidative damage to biomolecules including DNA,...
