High-grade gliomas may hijack signals from neurons to drive their own growth

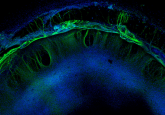
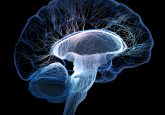
For the first time, researchers at Stanford University (CA, USA) have demonstrated that high-grade gliomas might integrate into the brain’s wiring. According to the team, high-grade gliomas form synapses that hijack electrical signals from healthy nerve cells to drive their own growth. In addition to this, interrupting these signals with an existing anti-epilepsy drug was demonstrated to greatly reduce the cancers’ growth in human tumors in mice. These findings have been published in Nature. How do high-grade gliomas hijack nerve cells? Within the study, the investigators determined that high-grade gliomas form synapses with healthy neurons, which can enable electrical signals...