November industry news: US FDA approves DANYELZA® for the treatment of neuroblastoma

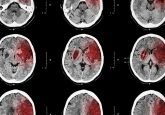
In this month’s industry news round-up, we hear more about the approval of multiple drugs for various diseases – from the US FDA approvals of DANYELZA® for neuroblastoma and BRILINTA® for stroke, to the approval of Fycompa® for epilepsy by the European Commission. We also hear how Aimovig® demonstrates superior results to topiramate in migraine prevention, in addition to a new partnership that aims to manufacture an experimental cell therapy for glioblastoma. Take a look at November’s headlines below: US FDA approves DANYELZA® for the treatment of neuroblastoma Aimovig® (erenumab) displays superior tolerability compared with topiramate in migraine prevention US...