Journal Watch: Alzheimer’s, diabetes and… dolphins?

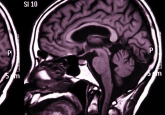
While Alzheimer’s disease (AD) and Type 2 diabetes mellitus (T2D) are two public health concerns in their own right, they are also frequently comorbid; linked by genetics, epidemiology and molecular biology. To date, both diseases are thought to be an inevitability of either modern civilisation or the length of human lifespan. However, a novel hypothesis put forward by Danièlle Gunn-Moore and colleagues in Alzheimer’s and Dementia challenges this view, suggesting that the convergent molecular antecendents to AD and T2D are caused by our post-reproductive longevity, not simply our increasing life span. The increase in AD and T2D can only be...