BACE1 levels are increased in plasma of Alzheimer’s disease patients compared with matched cognitively healthy controls
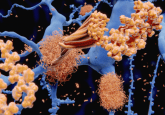
In Alzheimer's disease (AD), the amyloidogenic pathway results in the production of Aβ peptide from AβPP. Aβ has a hydrophobic nature and aggregates extracellularly, forming senile plaques [1]. In turn, neurofibrillary tangles are intracellular fibrillar aggregates of the hyperphosphorylated microtubule-associated Tau protein. Together, these entities are the key microscopic neuropathological hallmarks of AD [2]. In AD, the amyloidogenic pathway is the predominant route of AβPP cleavage, when it is first cleaved at the amino terminus by BACE1. This cleavage produces a large soluble secreted segment (sAβPPβ) and a membrane bound CTFβ of AβPP (also known as C99). Cleavage of CTFβ...