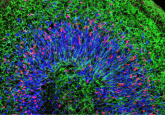
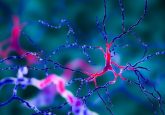
Contracting neuromuscular organoids have been developed
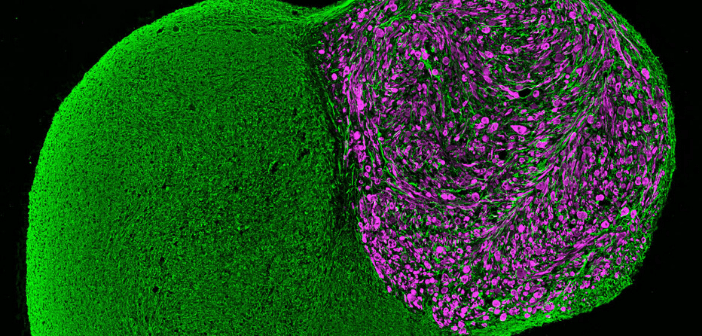
Credit: Jorge Miguel Faustino Martins, MDC
Functional neuromuscular organoids have been developed by researchers in the Gouti lab at the Max Delbrück Center for Molecular Medicine (MDC; Berlin, Germany). The team reported that these organoids can direct muscle tissue to contract by their ability to form complex neuronal networks, as they self-organize into spinal cord neurons and muscle tissue. The study, which has been published in Cell Stem Cell, paves the way forward for the study of human neuromuscular system development and disease. These neuromuscular organoids are an attractive model for studying neuromuscular disorders because they include functional neuromuscular junctions. In addition to this, these models...